Reproductive Endocrinology
8 Topics Diabetes Mellitus
24 Topics Calcium and Bone Metabolism
7 Topics Pituitary Disorders
2 Topics Lipidology and Obesity
13 Topics Thyroid Diseases
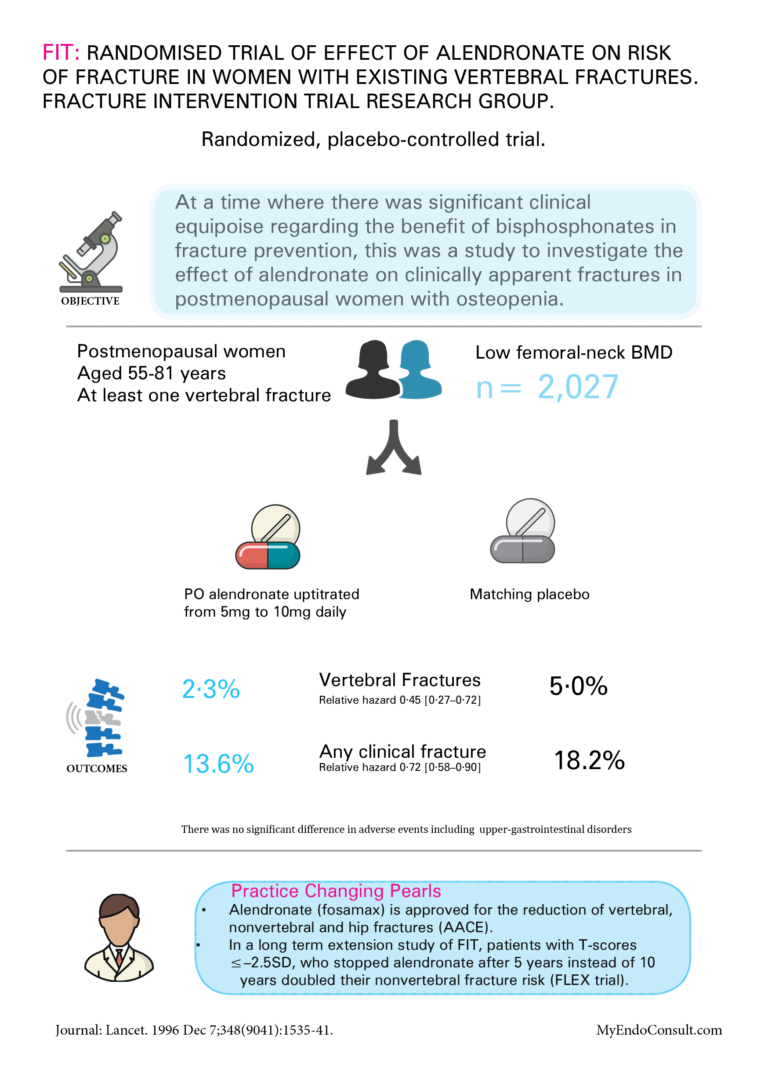
7 Topics FIT : Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group
FIT : Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group
• Alendronate (Fosamax) is approved for the reduction of vertebral, nonvertebral, and hip fractures (AACE).
• In a long-term extension study of FIT, patients with T-scores ≤–2.5SD, who stopped alendronate after 5 years instead of 10 years doubled their nonvertebral fracture risk (FLEX trial).
Infographic
Reference
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996 Dec 7;348(9041):1535-41.

