EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Preserved Ejection Fraction.
Review study protocol
Read Study Publication
Rationale and Clinical Equipoise
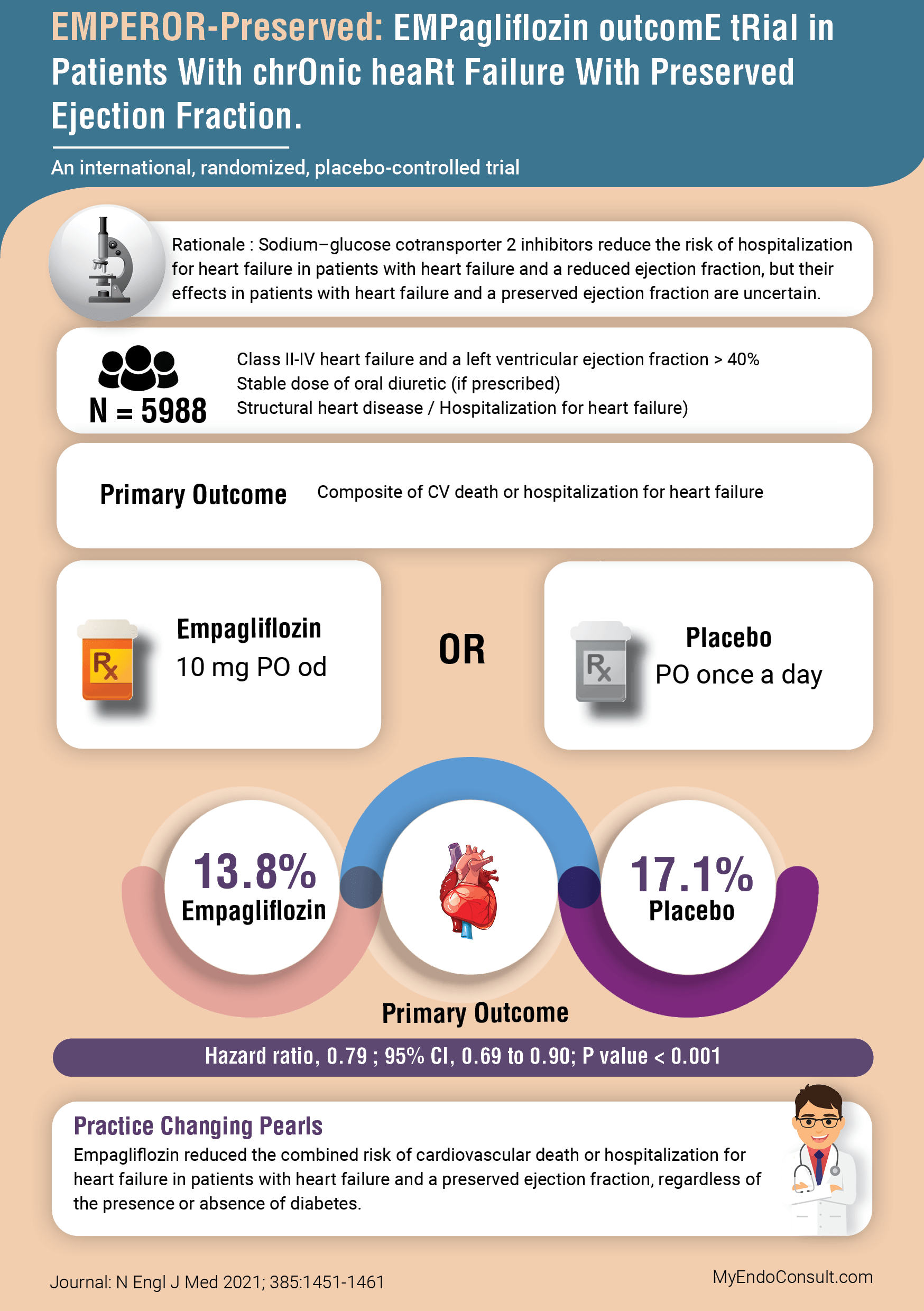
Sodium–glucose cotransporter 2 inhibitors reduce the risk of hospitalization for heart failure in patients with heart failure and a reduced ejection fraction, but their effects in patients with heart failure and a preserved ejection fraction are uncertain. SGLT2i address an important pathophysiologic defect in the ominous octet of type 2 diabetes mellitus, however the cardiovascular benefits was a welcome finding in the landmark EMPA-REG OUTCOME (empagliflozin) study from 2015. Subsequently, their cardiovascular related benefits was demonstrated in the EMPEROR-Reduced study from 2020 independent of the diabetes mellitus status of study participants with heart failure with reduced ejection fraction (HFrEF). There was however significant uncertainty regarding the benefits of SGLT2 inhibitors in heart failure with preserved ejection fraction. The Emperor Preserved study sought to elucidate the therapeutic benefit of empagliflozin in this patient population.
A multicenter, international, double-blind, randomized clinical trial. A total of 5988 subjects were randomized to empagliflozin (n=2997) or placebo (n=2991). The median duration of follow up was 26.2 months. An intention-to-treat analysis.
Study Eligibility Criteria
- Class II-IV heart failure and a left ventricular ejection fraction > 40%
- Stable dose of oral diuretic (if prescribed)
- Structural heart disease / Hospitalization for heart failure)
- BMI < 45 kg/m2
- Age >18 years
Primary Outcome
Composite of CV death or hospitalization for heart failure
Comparing Empagliflozin to Placebo 13.8% vs.17.1% (Hazard Ratio 0.79; 95% CI 0.69-0.90; P<0.001). The number needed to treat was 30
Secondary Outcomes
Hospitalizations for heart failure
8.6% vs.11.8% (Hazard Ratio of 0.71; 95% CI 0.60-0.83)
Death from all cardiovascular causes
7.3% vs.8.2% (Hazard Ratio of 0.91; 95% CI 0.76-1.09)
Critical Appraisal
Although the composite primary outcome, reached statistical significance, individual secondary outcomes resulted in discordant results. There was no statistically significant difference in deaths from cardiovascular causes when the empagliflozin group was compared to placebo. However, hospitalizations for heart failure were significantly lower in the intervention group compared to placebo. The primary outcome should therefore be interpreted with caution.
Practice Changing Pearls (Conclusion)
Empagliflozin reduced the combined risk of cardiovascular death or hospitalization for heart failure in patients with heart failure and a preserved ejection fraction, regardless of the presence or absence of diabetes.
References
Anker SD et al. EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021 Oct 14;385(16):1451-1461. doi: 10.1056/NEJMoa2107038. Epub 2021 Aug 27. PMID: 34449189. Redfield MM. Heart failure with preserved ejection fraction. New England Journal of Medicine. 2016;375(19):1868-1877.
Kindly Let Us Know If This Was helpful? Thank You!

