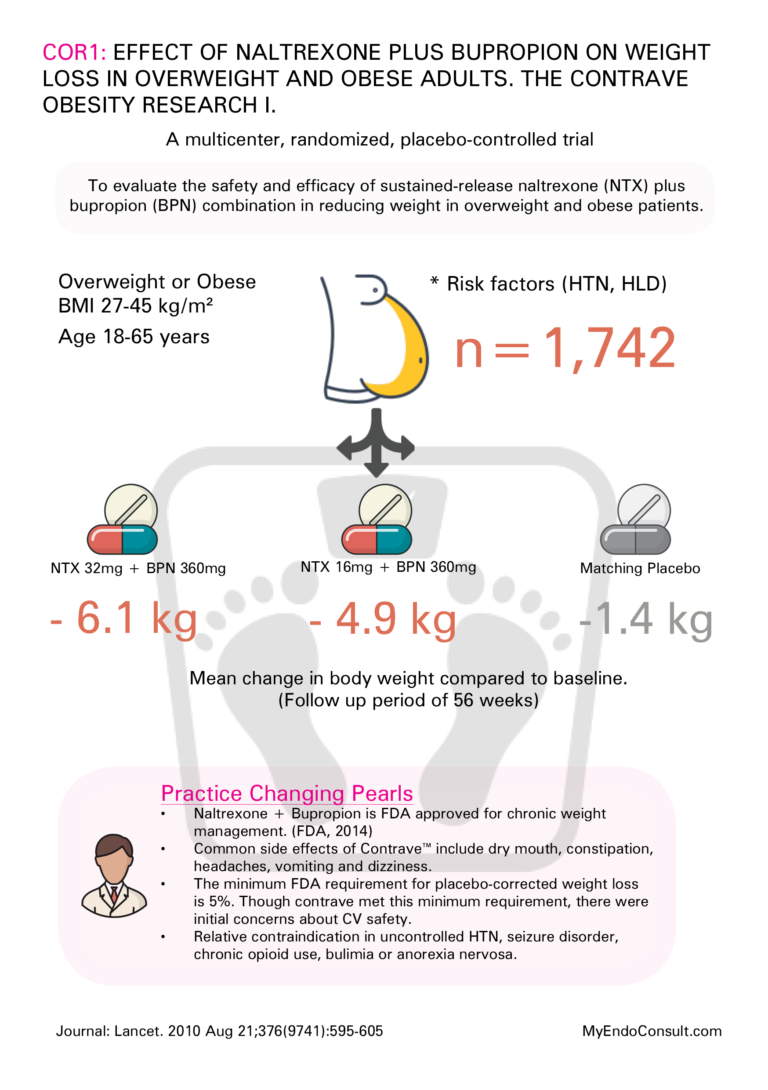
COR1
Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial
• Naltrexone + Bupropion is FDA approved for chronic weight management. (FDA, 2014)
• Common side effects of Contrave™ include dry mouth, constipation, headaches, vomiting, and dizziness.
• The minimum FDA requirement for placebo-corrected weight loss is 5%. Though contrave met this minimum requirement, there were initial concerns about CV safety.
• Relative contraindication in uncontrolled HTN, seizure disorder, chronic opioid use, bulimia, or anorexia nervosa.
Infographic
Reference
Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E; COR-I Study Group. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010 Aug 21;376(9741):595-605. doi: 10.1016/S0140-6736(10)60888-4. Epub 2010 Jul 29. Erratum in: Lancet. 2010 Aug 21;376(9741):594. Erratum in: Lancet. 2010 Oct 23;376(9750):1392.

